| |
p53 Information
p53 information
p53 story
p53 monoclonal antibodies
p53 pathways
p53 gene
mdm family
mouse models
ASPP family
|
mdm2 and mdm4 (mdmx)
Mdm2 discovery
The mdm2 gene
The mdm2 protein
The mdm2/p53 loop
The snp 309 in the mdm2 gene
mdm2/ versus mdm4 gene organisation
MDM2 (murine double minute 2) was first identified as the gene responsible for the spontaneous transformation of an immortalized murine cell line, BALB/c 3T3. The derivative cell line, 3T3-DM, contains 25–30 copies per cell of paired, acentric chromatin bodies called double minutes. Cell lines that overexpress MDM2 can form tumours in nude mice, although they do not have the ability to grow in soft agar or form foci. The genomic Mdm2 clone can also mediate immortalization of rat embryo fibroblasts (REFs) and transformation of REFs (as characterized by focus-forming ability and tumour formation in nude mice) in cooperation with an activated ras gene. Thus MDM2 acts as an oncogene in tissue culture systems.
Mdm2 discovery
 |
| |
 |
Barak, Y., and M. Oren (1992) EMBO J 11:2115-2121.
Enhanced binding of a 95 kDa protein to p53 in cells undergoing p53-mediated growth arrest.
To explore the biochemical functions of p53, we have initiated a search for cellular p53-binding proteins. Coprecipitation of three polypeptides was observed when cell lines overexpressing a temperature-sensitive (ts) p53 mutant were maintained at 32.5 degrees C (wild-type p53 activity, leading to growth arrest) but not at 37.5 degrees C (mutant p53 activity). One of these three proteins, designated p95 on the basis of its apparent molecular mass, was highly abundant in p53 immune complexes. We demonstrate herein that p95 is a p53-binding protein, which exhibits poor p53-binding in cells overproducing several distinct mutant p53 proteins. Yet, p95 associates equally well with both the wild-type (wt) and the mutant conformations of the ts p53 in transformed cells growth-arrested at 32.5 degrees C. On the basis of our findings we suggest that wt p53 activity increases p53-p95 complex formation and that such interaction may play a central role in p53 mediated tumour suppression. |
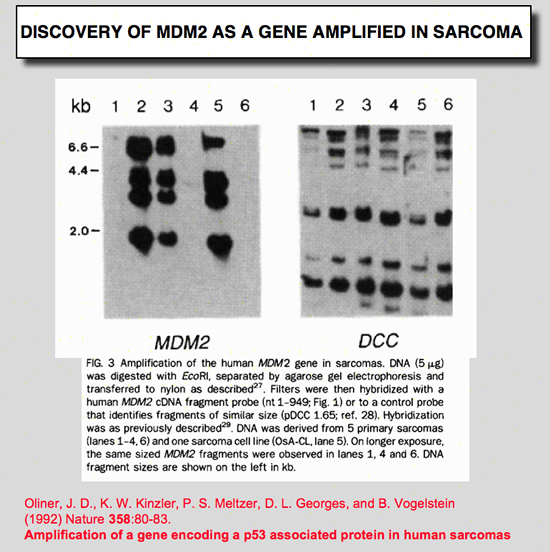
Oliner, J. D., K. W. Kinzler, P. S. Meltzer, D. L. George, and B. Vogelstein (1992) Nature 358:80-83.
Amplification of a gene encoding a p53-associated protein in human sarcomas.
Despite extensive data linking mutations in the p53 gene to human tumorigenesis, little is known about the cellular regulators and mediators of p53 function. MDM2 is a strong candidate for one such cellular protein; the MDM2 gene was originally identified by virtue of its amplification in a spontaneously transformed derivative of mouse BALB/c cells and the MDM2 protein subsequently shown to bind to p53 in rat cells transfected with p53 genes. To determine whether MDM2 plays a role in human cancer, we have cloned the human MDM2 gene. Here we show that recombinant-derived human MDM2 protein binds human p53 in vitro, and we use MDM2 clones to localize the human MDM2 gene to chromosome 12q13-14. Because this chromosomal position appears to be altered in many sarcomas, we looked for changes in human MDM2 in such cancers. The gene was amplified in over a third of 47 sarcomas, including common bone and soft tissue forms. These results are consistent with the hypothesis that MDM2 binds to p53, and that amplification of MDM2 in sarcomas leads to escape from p53-regulated growth control. This mechanism of tumorigenesis parallels that for virally-induced tumours, in which viral oncogene products bind to and functionally inactivate p53. |
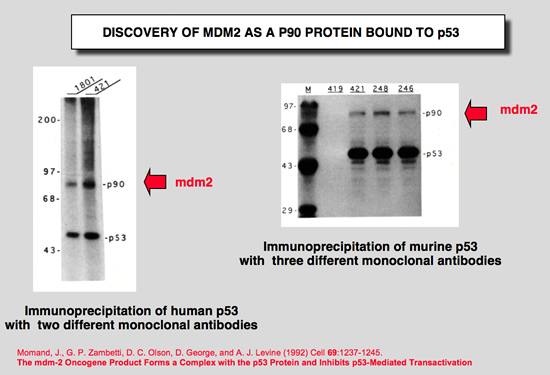
Momand, J., G. P. Zambetti, D. C. Olson, D. George, and A. J. Levine (1992) Cell 69:1237-1245.
The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation.
A cellular phosphoprotein with an apparent molecular mass of 90 kd (p90) that forms a complex with both mutant and wild-type p53 protein has been characterized, purified, and identified. The protein was identified as a product of the murine double minute 2 gene (mdm-2). The mdm-2 gene enhances the tumorigenic potential of cells when it is overexpressed and encodes a putative transcription factor. To determine if mdm-2 could modulate p53 transactivation, a p53-responsive element from the muscle creatine kinase gene was employed. A wild-type p53-expressing plasmid enhanced the expression of the p53-responsive element when cotransfected into cells that contain no endogenous p53. When a cosmid expressing mdm-2 was transfected with this p53-expressing plasmid, the transactivation of the p53-responsive element was inhibited. Thus, a product of the mdm-2 oncogene forms a tight complex with the p53 protein, and the mdm-2 oncogene can inhibit p53-mediated transactivation. |
The mdm2 gene
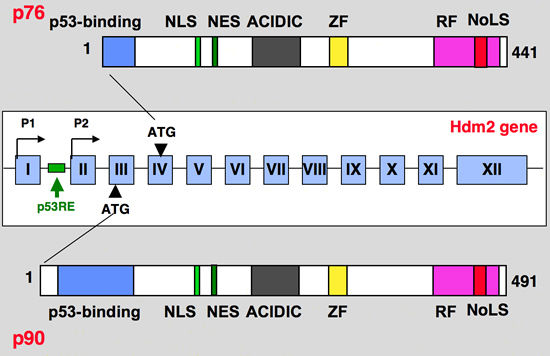 |
The hdm2 gene contains two transcriptional promoter elements termed P1 and P2. The upstream P1 promoter is utilized constitutively. When p53 binds to its sites located in the first intron of the Mdm2 gene, transcription of the P2 promoter, which is located downstream of the p53-binding sites, is induced. Activation of p53 in cells only mildly affects transcription of the upstream P1 promoter if at all, but highly induces P2 to produce a transcript that begins in the second exon. These two different Mdm2 transcripts have identical translational reading frames (the initiating AUG codon is in the third exon)
The products of the two mdm2 promoters appear to differ in another aspect of translation. mRNAs from both murine P1 and P2 promoters can direct the synthesis of at least two Mdm2 proteins. The larger protein is p90Mdm2, the inhibitor of p53. The smaller protein is p76Mdm2, an activator of p53 that lacks the first 49 amino acids of p90Mdm2. p76Mdm2 can be synthesized through internal initiation at AUG 50 in exon 4
Blue: p53 binding site
Light green, NLS: Nuclear Localization Signal
Dark Green, NES: Nuclear Exclusion Signal
Grey: Acidic region
Yellow, ZF: Zing finger domain
Pink, RF: Ring finger Domain
Red, NoLS
Nucleolar Localization Signal
|
The mdm2 protein
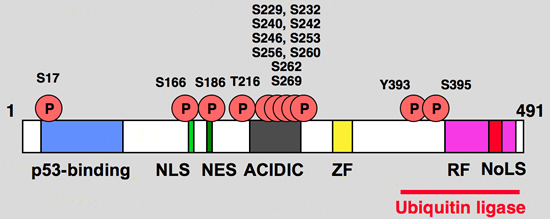 |
Functional domain and phosphorylation sites on the hdm2 protein
Blue: p53 binding site
Light green, NLS: Nuclear Localization Signal
Dark Green, NES: Nuclear Exclusion Signal
Grey: Acidic region
Yellow, ZF: Zing finger domain
Pink, RF: Ring finger Domain
Red, NoLS
Nucleolar Localization Signal |
mdm2 binding protein
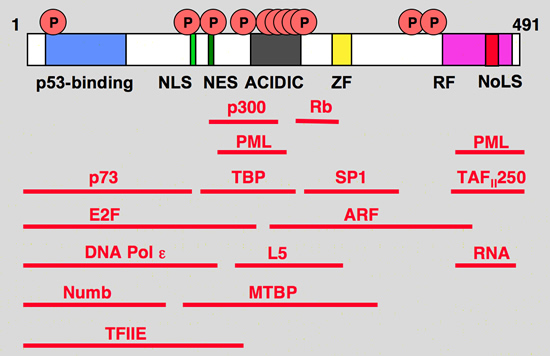 |
hdm2 interacting proteins
Blue: p53 binding site
Light green, NLS: Nuclear Localization Signal
Dark Green, NES: Nuclear Exclusion Signal
Grey: Acidic region
Yellow, ZF: Zing finger domain
Pink, RF: Ring finger Domain
Red, NoLS
Nucleolar Localization Signal |
The mdm2/p53 loop
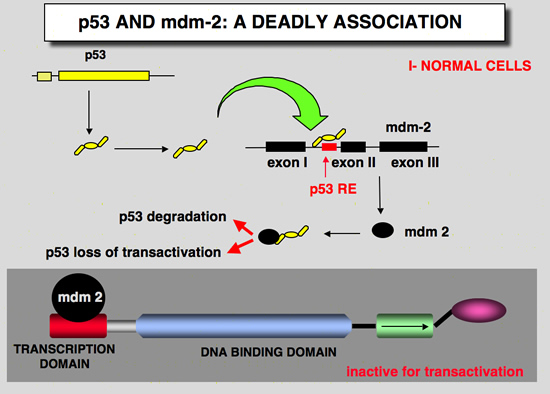 |
MDM2 is a RING-finger E3 ubiquitin ligase that specifically interacts with p53 through its N-terminal p53-binding domain. The C-terminal RING-finger motif of MDM2 not only promotes p53 ubiquitylation, but also targets MDM2 itself for auto-ubiquitylation and subsequent degradation.
In addition, MDM2 is also a negative regulator of p53-mediated transcriptional activity. The MDM2 gene is a transcriptional target of p53, so activation of the MDM2 gene by p53 would lead to repression of p53 activity.
The existence of this auto-regulatory feedback loop between MDM2 and p53 adds a complex feature to the p53–MDM2 pathway and makes MDM2 one of the most important regulators of p53 activity.
|
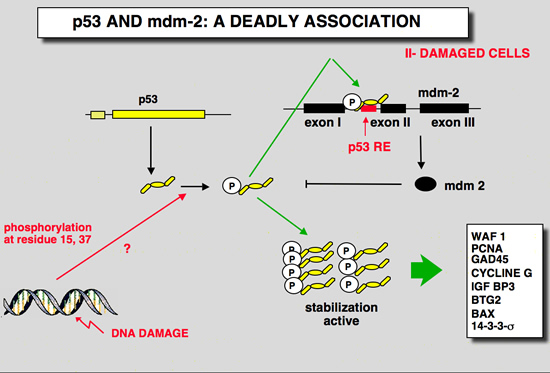 |
In cells that are exposed to various stress, interaction between p53 and mdm-2 is disrupted in large part due to posttranslational modifications of p53 and mdm-2.
More information on p53 pathways |
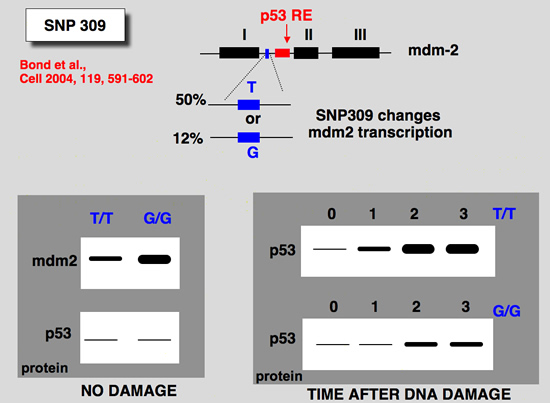
The snp 309 in the mdm2 gene
 |
| |
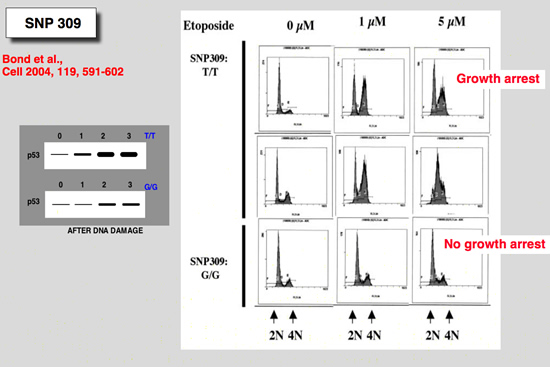 |
Bond, G. L., W. Hu, E. E. Bond, H. Robins, S. G. Lutzker, N. C. Arva, J. Bargonetti, F. Bartel, H. Taubert, P. Wuerl, K. Onel, L. Yip, S. J. Hwang, L. C. Strong, G. Lozano, and A. J. Levine (2004) Cell 119:591-602.
A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans
The tumor suppressor p53 gene is mutated in minimally half of all cancers. It is therefore reasonable to assume that naturally occurring polymorphic genetic variants in the p53 stress response pathway might determine an individual's susceptibility to cancer. A central node in the p53 pathway is the MDM2 protein, a direct negative regulator of p53. In this report, a single nucleotide polymorphism (SNP309) is found in the MDM2 promoter and is shown to increase the affinity of the transcriptional activator Sp1, resulting in higher levels of MDM2 RNA and protein and the subsequent attenuation of the p53 pathway. In humans, SNP309 is shown to associate with accelerated tumor formation in both hereditary and sporadic cancers. A model is proposed whereby SNP309 serves as a rate-limiting event in carcinogenesis. |
mdm2/ versus mdm4 gene organisation
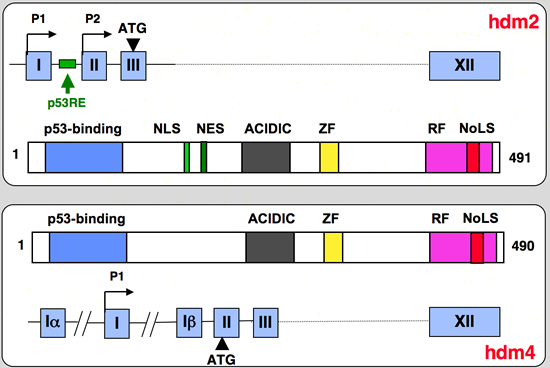 |
mdm2 (human or mouse) versus mdm4 (mouse) gene and protein structure
Marine, J. C., and Jochemsen, A. G. (2004). Mdmx and Mdm2: brothers in arms? Cell Cycle 3, 900-904.
Marine, J. C., and Jochemsen, A. G. (2005). Mdmx as an essential regulator of p53 activity. Biochem Biophys Res Commun 331, 750-760. |
|
|






