| |
p53 Information
p53 information
p53 story
p53 monoclonal antibodies
p53 pathways
p53 gene
mdm family
mouse models
ASPP family
|
ASPP family (iASPP, ASPP1, ASPP2)
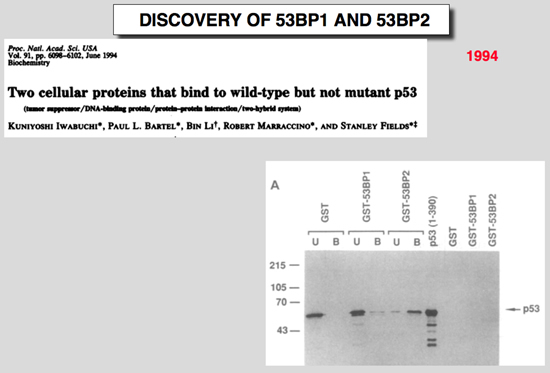 |
Iwabuchi, K., P. L. Bartel, B. Li, R. Marraccino, and S. Fields (1994) Proc Natl Acad Sci U S A 91:6098-6102.
Two cellular proteins that bind to wild-type but not mutant p53.
p53 is a tumor-suppressor protein that can activate and repress transcription. Using the yeast two-hybrid system, we identified two previously uncharacterized human proteins, designated 53BP1 and 53BP2, that bind to p53. 53BP1 shows no significant homology to proteins in available databases, whereas 53BP2 contains two adjacent ankyrin repeats and a Src homology 3 domain. In vitro binding analyses indicate that both of these proteins bind to the central domain of p53 (residues 80-320) required for site-specific DNA binding. Consistent with this finding, p53 cannot bind simultaneously to 53BP1 or 53BP2 and to a DNA fragment containing a consensus p53 binding site. Unlike other cellular proteins whose binding to p53 has been characterized, both 53BP1 and 53BP2 bind to the wild-type but not to two mutant p53 proteins identified in human tumors, suggesting that binding is dependent on p53 conformation. The characteristics of these interactions argue that 53BP1 and 53BP2 are involved in some aspect of p53-mediated tumor suppression. |
|
Nakagawa, H., K. Koyama, Y. Murata, M. Morito, T. Akiyama, and Y. Nakamura (2000) Cancer Res S 60:101-105.
APCL, a central nervous system-specific homologue of adenomatous polyposis coli tumor suppressor, binds to p53-binding protein 2 and translocates it to the perinucleus.
APCL, a central nervous system-specific sequence homologue of the adenomatous polyposis coli tumor suppressor, can regulate the cytoplasmic level of beta-catenin as the adenomatous polyposis coli tumor suppressor does, but its overall biological function remains unclear. Using a yeast two-hybrid system, we attempted to isolate proteins that might associate with the unique COOH-terminus of APCL. Among 166 cDNA clones isolated from a human fetal-brain cDNA library as candidates for interaction with APCL, 32 encoded parts of p53-binding protein 2 (53BP2), a molecule that interacts with p53 and Bcl2. An in vitro binding assay indicated that the Src-homology-3 domain and the ankyrin-repeat domain of 53BP2 were both required for binding to the COOH-terminus of APCL. Confocal microscopy showed that APCL and 53BP2 proteins were localized together in the perinuclei of normal mammalian cells, but this was not the case in cells that expressed truncated APCL and 53BP2 proteins. These findings suggested that binding of the COOH-terminus of APCL to 53BP2 regulates the cytoplasmic location of 53BP2. Because 53BP2 also interacts with p53 and Bcl2 and regulates p53 function, our results suggest that APCL might be involved in the p53/Bcl2-linked pathway of cell-cycle progression and cell death.
Chen, D., E. Padiernos, F. Ding, I. S. Lossos, and C. D. Lopez (2005) Cell Death Differ 12:358-368.
Apoptosis-stimulating protein of p53-2 (ASPP2/53BP2L) is an E2F target gene.
The p53 pathway is a central apoptotic regulator. Deregulation of the Rb/E2F pathway occurs in a majority of tumors, resulting in both unrestrained proliferation and enhanced apoptosis sensitivity via p53-dependent and independent mechanisms. However, the mechanisms coupling the p53 and Rb/E2F pathways remain incompletely understood. We report that ASPP2/53BP2L, a p53/p73-binding protein that promotes p53/p73-dependent apoptosis, is an E2F target gene. The ASPP2/53BP2L promoter was identified and ectopic expression of transcription-competent E2F-1 (E2F-2 and E2F-3) stimulated an ASPP2/53BP2L promoter-luciferase reporter. Mutational analysis of the ASPP2/53BP2L promoter identified E2F-binding sites that cooperate for E2F-1 induction and basal repression of ASPP2/53BP2L. Moreover, endogenous ASPP2/53BP2L levels increased after E2F-1 expression, and E2F-1 bound the endogenous ASPP2/53BP2L promoter after chromatin immunoprecipitation. Typical for an E2F target, ASPP2/53BP2L expression was maximal in early S-phase. Thus, ASPP2/53BP2L is downstream of E2F, suggesting that it functions as a common link between the p53/p73 and Rb/E2F apoptotic pathways.
|
|
Bergamaschi, D., Y. Samuels, N. J. O'Neil, G. Trigiante, T. Crook, J. K. Hsieh, D. J. O'Connor, S. Zhong, I. Campargue, M. L. Tomlinson, P. E. Kuwabara, and X. Lu (2003) Nat Genet 33:162-167.
iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human.
We have previously shown that ASPP1 and ASPP2 are specific activators of p53; one mechanism by which wild-type p53 is tolerated in human breast carcinomas is through loss of ASPP activity. We have further shown that 53BP2, which corresponds to a C-terminal fragment of ASPP2, acts as a dominant negative inhibitor of p53 (ref. 1). Hence, an inhibitory form of ASPP resembling 53BP2 could allow cells to bypass the tumor-suppressor functions of p53 and the ASPP proteins. Here, we characterize such a protein, iASPP (inhibitory member of the ASPP family), encoded by PPP1R13L in humans and ape-1 in Caenorhabditis elegans. iASPP is an evolutionarily conserved inhibitor of p53; inhibition of iASPP by RNA-mediated interference or antisense RNA in C. elegans or human cells, respectively, induces p53-dependent apoptosis. Moreover, iASPP is an oncoprotein that cooperates with Ras, E1A and E7, but not mutant p53, to transform cells in vitro. Increased expression of iASPP also confers resistance to ultraviolet radiation and to cisplatin-induced apoptosis. iASPP expression is upregulated in human breast carcinomas expressing wild-type p53 and normal levels of ASPP. Inhibition of iASPP could provide an important new strategy for treating tumors expressing wild-type p53.
|
|
Gorina, S., and N. P. Pavletich (1996) Science 274:1001-1005.
Structure of the p53 tumor suppressor bound to the ankyrin and SH3 domains of 53BP2.
Mutations in the p53 tumor suppressor are among the most frequently observed genetic alterations in human cancer and map to the 200-amino acid core domain of the protein. The core domain contains the sequence-specific DNA binding activity and the in vitro 53BP2 protein binding activity of p53. The crystal structure of the p53 core domain bound to the 53BP2 protein, which contains an SH3 (Src homology 3) domain and four ankyrin repeats, revealed that (i) the SH3 domain binds the L3 loop of p53 in a manner distinct from that of previously characterized SH3-polyproline peptide complexes, and (ii) an ankyrin repeat, which forms an L-shaped structure consisting of a beta hairpin and two alpha helices, binds the L2 loop of p53. The structure of the complex shows that the 53BP2 binding site on the p53 core domain consists of evolutionarily conserved regions that are frequently mutated in cancer and that it overlaps the site of DNA binding. The six most frequently observed p53 mutations disrupt 53BP2 binding in vitro. The structure provides evidence that the 53BP2-p53 complex forms in vivo and may have a critical role in the p53 pathway of tumor suppression.
|
|
|
Samuels-Lev, Y., D. J. O'Connor, D. Bergamaschi, G. Trigiante, J. K. Hsieh, S. Zhong, I. Campargue, L. Naumovski, T. Crook, and X. Lu (2001) Mol Cell 8:781-794.
ASPP proteins specifically stimulate the apoptotic function of p53.
We identified a family of proteins termed ASPP. ASPP1 is a protein homologous to 53BP2, the C-terminal half of ASPP2. ASPP proteins interact with p53 and specifically enhance p53-induced apoptosis but not cell cycle arrest. Inhibition of endogenous ASPP function suppresses the apoptotic function of endogenous p53 in response to apoptotic stimuli. ASPP enhance the DNA binding and transactivation function of p53 on the promoters of proapoptotic genes in vivo. Two tumor-derived p53 mutants with reduced apoptotic function were defective in cooperating with ASPP in apoptosis induction. The expression of ASPP is frequently downregulated in human breast carcinomas expressing wild-type p53 but not mutant p53. Therefore, ASPP regulate the tumor suppression function of p53 in vivo. |
|
Bergamaschi, D., Y. Samuels, B. Jin, S. Duraisingham, T. Crook, and X. Lu (2004) Mol Cell Biol 24:1341-1350.
ASPP1 and ASPP2: common activators of p53 family members.
We recently showed that ASPP1 and ASPP2 stimulate the apoptotic function of p53. We show here that ASPP1 and ASPP2 also induce apoptosis independently of p53. By binding to p63 and p73 in vitro and in vivo, ASPP1 and ASPP2 stimulate the transactivation function of p63 and p73 on the promoters of Bax, PIG3, and PUMA but not mdm2 or p21(WAF-1/CIP1). The expression of ASPP1 and ASPP2 also enhances the apoptotic function of p63 and p73 by selectively inducing the expression of endogenous p53 target genes, such as PIG3 and PUMA, but not mdm2 or p21(WAF-1/CIP1). Removal of endogenous p63 or p73 with RNA interference demonstrated that (16) the p53-independent apoptotic function of ASPP1 and ASPP2 is mediated mainly by p63 and p73. Hence, ASPP1 and ASPP2 are the first two identified common activators of all p53 family members. All these results suggest that ASPP1 and ASPP2 could suppress tumor growth even in tumors expressing mutant p53.
|
|
|
Bergamaschi, D., Y. Samuels, N. J. O'Neil, G. Trigiante, T. Crook, J. K. Hsieh, D. J. O'Connor, S. Zhong, I. Campargue, M. L. Tomlinson, P. E. Kuwabara, and X. Lu (2003) Nat Genet 33:162-167.
iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human.
We have previously shown that ASPP1 and ASPP2 are specific activators of p53; one mechanism by which wild-type p53 is tolerated in human breast carcinomas is through loss of ASPP activity. We have further shown that 53BP2, which corresponds to a C-terminal fragment of ASPP2, acts as a dominant negative inhibitor of p53 (ref. 1). Hence, an inhibitory form of ASPP resembling 53BP2 could allow cells to bypass the tumor-suppressor functions of p53 and the ASPP proteins. Here, we characterize such a protein, iASPP (inhibitory member of the ASPP family), encoded by PPP1R13L in humans and ape-1 in Caenorhabditis elegans. iASPP is an evolutionarily conserved inhibitor of p53; inhibition of iASPP by RNA-mediated interference or antisense RNA in C. elegans or human cells, respectively, induces p53-dependent apoptosis. Moreover, iASPP is an oncoprotein that cooperates with Ras, E1A and E7, but not mutant p53, to transform cells in vitro. Increased expression of iASPP also confers resistance to ultraviolet radiation and to cisplatin-induced apoptosis. iASPP expression is upregulated in human breast carcinomas expressing wild-type p53 and normal levels of ASPP. Inhibition of iASPP could provide an important new strategy for treating tumors expressing wild-type p53. |
|
|






