


 |
|||||||
 |
 |
||||||
Our WorkXenopus p53
|
Xenopus p53Cloning and characterization of xenopus p53 cDNA
Cloning and characterization of xenopus p53 cDNAThe xenopus p53 cDNA was cloned and sequenced in 1987 (Soussi et al., Oncogene, 1987). Sequence comparison with murine and human p53 shows that conserved sequences in these proteins are not uniformly distributed along the molecule, allowing five highly conserved domains to be defined. These domains do not appear on alignment of mammalian p53. (HCD I to V , figure 1).
DNA damage induces the stabilization of Xp53 in A6 cell lineCell line A6 was developed by Raferty from X. laevis adult kidney (Rafferty, 1969). This non-tumoral epithelioma cell line has been extensively used for physiological studies. We focussed on the biological activity of the Xp53 in this cell line, including stabilisation and cell cycle arrest after DNA damage. The stabilisation of endogenous Xp53 was detected by western blot using specific polyclonal and monoclonal antibodies.
DNA damage induces apoptosis of Xp53 in A6 cell line
Xenopus p53 binds to human p53 DNA response element
|
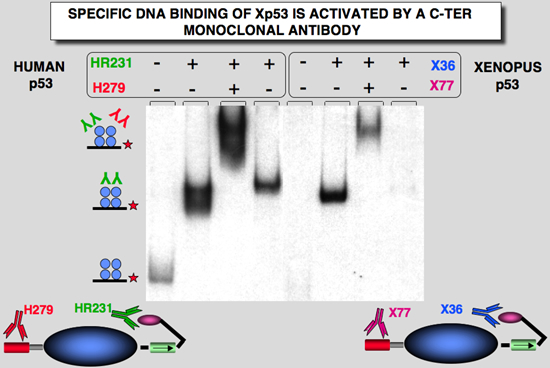 |
Figure 4: Activation of sequence-specific DNA binding of Hp53and Xp53. Activation and DNA binding experiments were analysed by gel-shift assay. Effect of various mAbs on DNA binding activity. The probe used in this experiment was Human Waf1. |
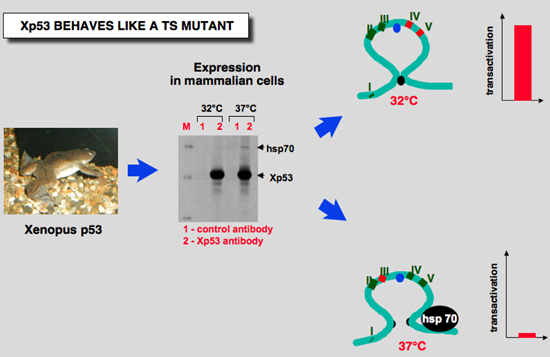 |
Figure 5: Xenopus p53 behaves like a ts mutant at 37°C |
Human p53 protein (Hp53) can be divided into five domains, each corresponding to specific functions (Figure 6) . I) The amino-terminus part 1-39 contains the acidic transactivation domain and the mdm2 protein binding site. II) Region 40-100 contains series repeated proline residues that are conserved in the majority of p53. III) The central region (101-306) contains the DNA binding domain. It is the target of 90% of p53 mutations found in human cancers. IV) The oligomerization domain (307-355) consists of a beta-strand, followed by an alpha-helix necessary for dimerization, as p53 is composed of a dimer of two dimers. A nuclear export signal is localized in this oligomerization domain. V) The carboxy-terminus of p53 (356-393) contains 3 nuclear localization signals and a non-specific DNA binding domain that binds to damaged DNA. This region is also involved in downregulation of DNA binding of the central domain.
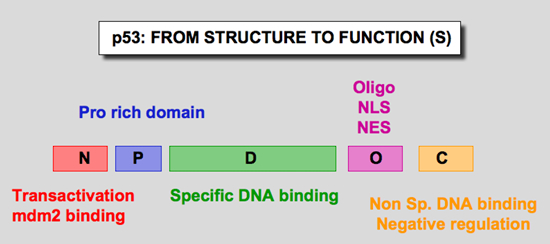 |
| Figure 6: Domains of the p53 protein |
We constructed two artificial p53 minigenes in order to perform swapping experiments between human and Xenopus p53 (Figure 7). Five cassettes, each corresponding to a functional domain described above, were devised. For easy swapping, each cassette was designed as an unique exon flanked by an artificial intron. Unique restriction sites were introduced in each intron so that each p53 cassette could be easily moved from one construct to another. These two constructs allow many swapping experiments in order to generate various hybrid proteins.
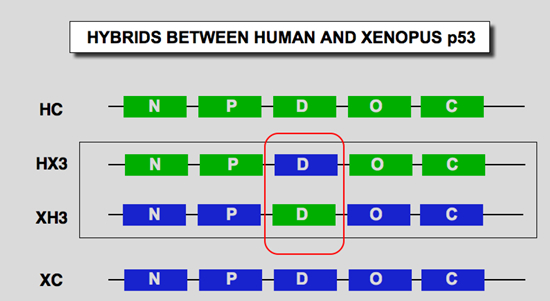 |
| Figure 7: structure of the artificial p53 gene divided into 5 exons. The boundary of each domain in amino acid residues is given for human (Hp53) and Xenopus p53 (Xp53); N: transactivation domain; P: proline-rich domain; D; DNA binding domain; O: oligomerization domain; C; carboxy-terminal regulation domain. HC: Human p53 protein; XC: Xenopus p53; HX3: Human p53 with Xp53 DNA Binding domain; XH3: Xp53 with Hp53 DNA binding domain. |
At 32°C, both wild Hp53 and Xp53 were active and transactivated the various promoters (figure 8). The efficiency of the two proteins was similar. At 37°C, Xp53 was completely inactive. Western blot experiments indicated that these observations are not related to a lack of expression of the various proteins, as expression of each protein was higher at 37°C, supporting our data concerning the temperature-sensitivity of frog p53 (Figure 8). XH3, the Hp53 DNA binding domain inside the Xp53 protein, had a similar behavior to that of Hp53 at both temperatures. Introduction of the human DNA binding domain into a Xenopus framework therefore restores normal p53 behavior, suggesting that temperature-sensitivity is linked to this region. This observation is confirmed by the behavior of HX3, a human p53 with the Xp53 DNA binding domain. This mutant is inactive at 37°C and partially or fully active at 32°C. Swapping another region of p53 (cassette P) had no effect on temperature-sensitivity
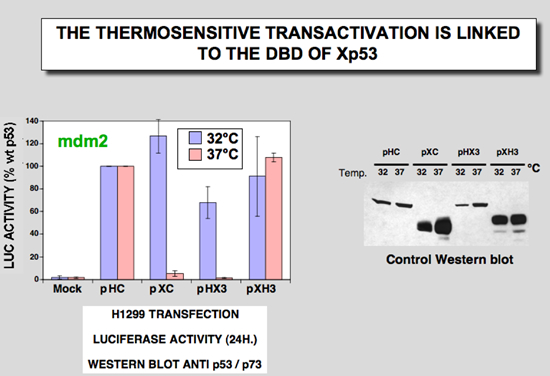 |
Figure 8: Exponentially growing Saos-2 cells were cotransfected with 50 ng of p53 expression vector and 1 microg of reporter plasmid. Luciferase assays were performed 24 h after transfection. Results are expressed as percent of activation relative to wt p53 used under the same experimental conditions (32 °C or 37 °C). Expression of wt p53 at 32 °C results in a 20 –50% decrease in activity compared with 37 °C, depending on the promoter used. B, equal amounts of protein extract were analyzed by Western blot with X77 monoclonal antibody to check expression. X77 recognizes both Hp53 and Xp53 with a higher affinity for Xp53. Only the 32 °C/37 °C ratio of each protein can therefore be assessed in this experiment |
The ability of the various constructs to mediate apoptosis was evaluated in H1299 cells using a transient transfection assay. The two proteins with the human p53 DNA binding domain (Hp53 and XH3) were both able to induce apoptosis at 37°C, whereas the two proteins with the Xenopus p53 DNA binding domain were unable to induce apoptosis and showed a similar behavior to that of mutant p53His273 (Figure 9)
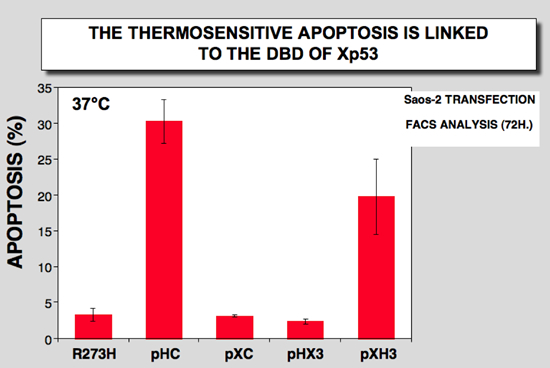 |
Figure 9: 100-mm Petri dishes with H1299 cells were transfected with 10 microg. of DNA. Apoptosis was analyzed 72 h after transfection |
The conformational change can be detected by recognition by cellular chaperone hsp70. Immunoprecipitation of Xp53 expressed at 37°C led to the detection of Xp53 (46 kDa) and co-precipitation of a 70-kDa protein (Figure 10). This co-precipitation was not detected at 32°C nor when wild-type human p53 was expressed at either temperature (Figure 10). Control experiments with the human mutant p53His175 indicated that it binds to hsp70 at both temperatures. This observation, previously described in 1989, led us to the hypothesis that the conformation of Xp53 is altered at 37°C (32). The identity of the 70-kDa protein was confirmed by immunoprecipitation directed against p53 followed by Western blot with hsp70 antibody (Fig. 2C). Using similar experimental conditions in H1299 cells, we showed that HX3 protein coprecipitates with hsp70 only at 37°C, whereas XH3 did not show any interaction at either temperature. Control immunoblot shows that a similar amount of each protein was used for each temperature (Figure 10). These observations suggest that the central region of p53 is important for the conformational change of Xp53.
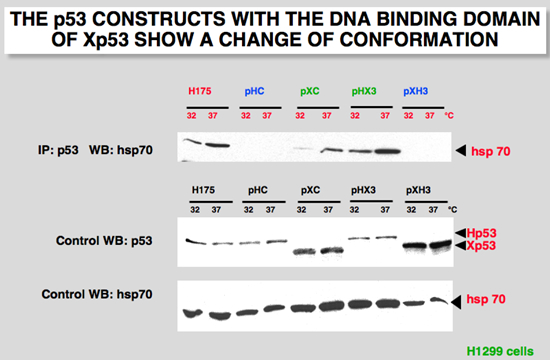 |
Figure 10:H1299 cells were cotransfected with 5 microg. of the indicated plasmid. Cells were incubated at either 30 or 37 °C and extracted 48 h after transfection. Unlabeled cell extracts were immunoprecipitated with p53 antibody, DO7 or X36 for human and Xenopus p53, respectively. After immunoprecipitation and electrophoresis, the proteins were transferred onto a membrane that was probed with a specific hsp70 antibody |
The four constructs described above were cotransfected into H1299 cells with an expression vector specific for p73. Mutant p53His175 was used as a positive control, as it undergoes a drastic conformational change and efficiently binds to p73 (25-27). The transfection experiment was performed at both 30°C and 37°C. We performed all our experiments with a milder NP40 buffer. Under these conditions, wild-type p53 did not co-precipitate efficiently with p73 (Fig. 11A, pHC). The human mutant p53His175 bound efficiently to p73 at both temperatures (Fig. 11A, H175). The behavior of Xp53 indicates that it bound more efficiently to p73 at 37°C than at 30°C, suggesting a temperature-sensitive behavior for this activity (Fig. 5A, pXC). Hybrid protein HX3 with the Xp53 DNA binding site behaved in a similar way, while the behavior of the other protein, XH3, was similar to that of Hp53. As indicated by the control Western blot, the same amount of p53 and p73 was expressed and used for all these experiments (Fig. 11B and 11C). This interaction was also analyzed in insect cells. Recombinant baculoviruses were used to express the various recombinant proteins in cells infected at 28°C. In this system, only mutant p53His175 bound efficiently to p73, whereas wt Hp53 and Xp53 did not.
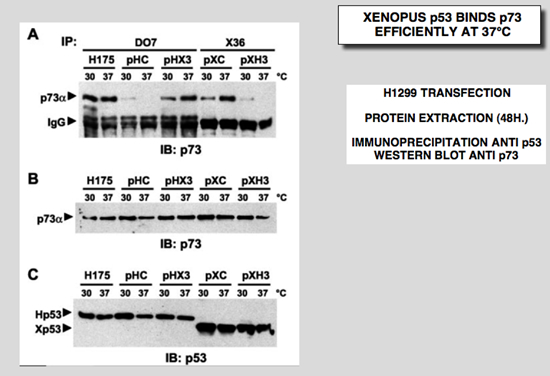 |
Figure 11: Hybrid p53 proteins with change of conformation associate with p73. H1299 cells were cotransfected with 2.5 mg of p73-Ha tagged expression vector and 2.5 mg of the indicated plasmid. Cells were incubated at either 30 or 37°C and extracted 48 h after transfection. Before immunoprecipitation, the amount of p53 was normalized by Western blot to ensure immunoprecipitation with an equivalent amount. A: cell extracts were immunoprecipitated with a p53 monoclonal antibody (DO7 for p53His175, Hp53 and HX3; X36 for Xp53 and XH3). After immunoprecipitation and electrophoresis, the proteins were transferred onto a membrane that was probed with an anti-Ha antibody for p73 detection. B and C: total extracts were analyzed by Western blot with the Ha-tag (p73) and X77 (Hp53 and Xp53) monoclonal antibodies, respectively. D: Sf9 cells were co-infected with various recombinant baculoviruses for 48 h. Cell extracts were incubated at either 4°C or 40°C for 15 min before immunoprecipitation with a p53 antibody (DO7 or X36). After immunoprecipitation and electrophoresis, the proteins were transferred onto a membrane that was probed with a specific p73 antibody. Control Western blot with total extract was probed directly with X77 that recognized Hp53, Xp53 and p73. E: H1299 cells were cotransfected with 2.5 mg of p73-Ha tagged expression vector and 2.5 mg of the indicated plasmid. Cells were incubated at either 32°C or 37°C and extracted 48 h after transfection. Cell extracts were immunoprecipitated with a p53 monoclonal antibody or a polyclonal sera that reacts with the amino-terminus of Xp53. After immunoprecipitation and electrophoresis, the proteins were transferred onto a membrane that was probed with an anti-Ha antibody for p73 detection and a mix of X77 and DO7 for p53 detection |
Bensaad, K., Le Bras, M., Unsal, K., Strano, S., Blandino, G., Tominaga, O., Rouillard, D. and Soussi T (2003) Change of Conformation of the DNA-binding Domain of p53 Is the Only Key Element for Binding of and Interference with p73. J Biol Chem, 278, 10546-10555.
Download the pdf
Bensaad, K., Rouillard, D. and Soussi T. (2001) Regulation of the cell cycle by p53 after DNA damage in an amphibian cell line. Oncogene, 20, 3766-3775.
Download the pdf
Hardy-Bessard AC, Garay E, Lacronique V, Legros Y, Demarquay C, Houque A, Portefaix JM, Granier C and Soussi T (1998) Regulation of the specific DNA binding activity of Xenopus laevis p53: evidence for conserved regulation through the carboxy-terminus of the protein. Oncogene 16: 883-890.
Download the pdf
 |
Home | Our Work |p53 Info| p53 Database | p53 Link | Contact us |