| |
p53 Information
p53 information
p53 story
p53 monoclonal antibodies
p53 pathways
p53 gene
mdm family
mouse models
|
p53 KO
Go back to the list
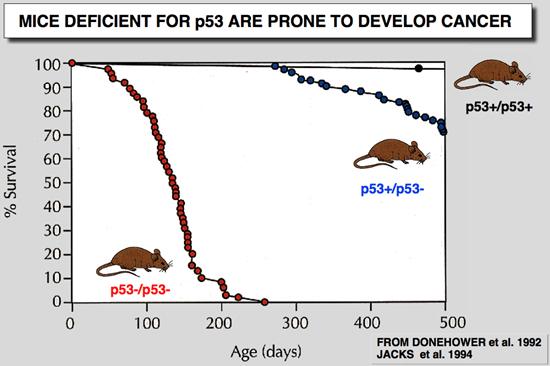 |
Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. J. Montgomery, J. S. Butel, and A. Bradley (1992) Nature 356:215-221.
Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours.
Mutations in the p53 tumour-suppressor gene are the most frequently observed genetic lesions in human cancers. To investigate the role of the p53 gene in mammalian development and tumorigenesis, a null mutation was introduced into the gene by homologous recombination in murine embryonic stem cells. Mice homozygous for the null allele appear normal but are prone to the spontaneous development of a variety of neoplasms by 6 months of age. These observations indicate that a normal p53 gene is dispensable for embryonic development, that its absence predisposes the animal to neoplastic disease, and that an oncogenic mutant form of p53 is not obligatory for the genesis of many types of tumours. |
Jacks, T., L. Remington, B. O. Williams, E. M. Schmitt, S. Halachmi, R. T. Bronson, and R. A. Weinberg (1994) Curr Biol 4:1-7.
Tumor spectrum analysis in p53-mutant mice
BACKGROUND: The p53 tumor suppressor gene is mutated in a large percentage of human malignancies, including tumors of the colon, breast, lung and brain. Individuals who inherit one mutant allele of p53 are susceptible to a wide range of tumor types. The gene encodes a transcriptional regulator that may function in the cellular response to DNA damage. The construction of mouse strains carrying germline mutations of p53 facilitates analysis of the function of p53 in normal cells and tumorigenesis. RESULTS: In order to study the effects of p53 mutation in vivo, we have constructed a mouse strain carrying a germline disruption of the gene. This mutation removes approximately 40% of the coding capacity of p53 and completely eliminates synthesis of p53 protein. As observed previously for a different germline mutation of p53, animals homozygous for this p53 deletion mutation are viable but highly predisposed to malignancy. Heterozygous animals also have an increased cancer risk, although the distribution of tumor types in these animals differs from that in homozygous mutants. In most cases, tumorigenesis in heterozygous animals is accompanied by loss of the wild-type p53 allele. CONCLUSION: We reaffirm that p53 function is not required for normal mouse development and conclude that p53 status can strongly influence tumor latency and tissue distribution. |
|
|






